Organization chart
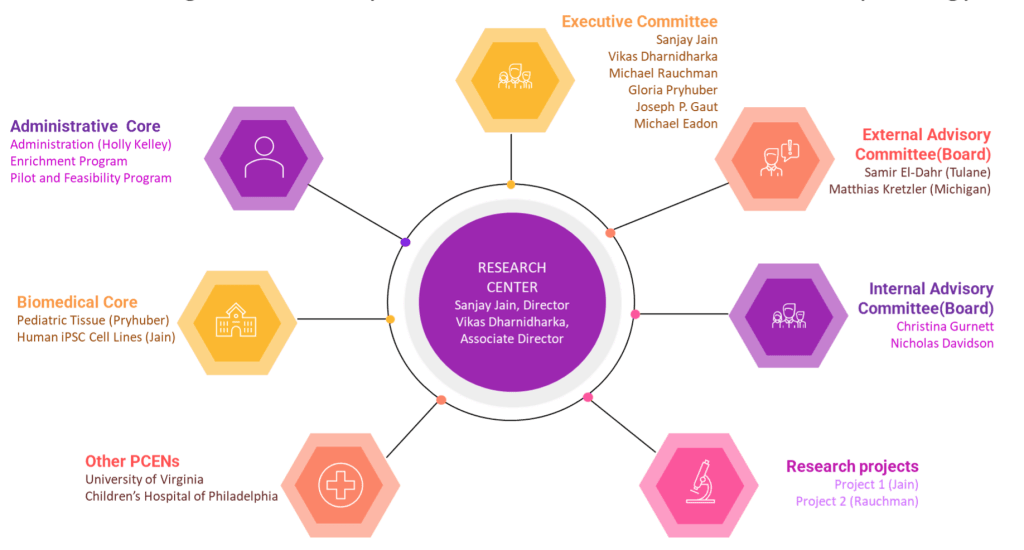
Executive committee
Director

Sanjay Jain, MD, PhD
Professor of Medicine, Nephrology
Professor of Pediatrics, Molecular Genetics and Genomics Program
Director Kidney Translational Research Center
Washington University in St. Louis School of Medicine
Sanjay Jain is a Professor of Medicine, Pediatrics and Pathology & Immunology at the Washington University School of Medicine in St. Louis, Missouri, USA (WUSM). His laboratory focuses on how kidneys and the lower urinary tract develop and organize to maintain homeostasis across lifespan in health and disease. His has defined key developmental pathways and mechanisms that regulate the joining of primitive ureter and bladder, initiation of the collecting system and branching morphogenesis of the kidney and genetic mutations associated with CAKUT. He leads multiple NIH-sponsored atlas efforts to map healthy and disease states in the human kidney including HuBMAP, KPMP, RBK/GUDMAP and Pediatric Center of Excellence in Nephrology. The team has identified, validated and mapped ~100 cell identities in the kidney including healthy and injured cells and defined genes and pathways that help recovery or predict decline in kidney function.
Associate director

Vikas Dharnidharka, MD
Professor of Pediatrics, Nephrology
Division Director, Pediatric Nephrology
Washington University in St. Louis School of Medicine
Vikas Dharnidharka is a patient-oriented researcher with interests in chronic renal failure, pediatric kidney transplantation and post-transplant infections. He perform epidemiological analyses of very large national databases to elucidate risk factors for events and the outcomes after events, typically infectious events. These large databases include UNOS, USRDS, NAPRTCS. HE also participate in multi-center clinical and mechanistic research trials in the areas of chronic renal insufficiency, dialysis and transplantation. His work gets funding from NIH institutes (NIDDK, NIAID) and industry. He is particularly known for my work related to a post-transplant malignancy called post-transplant lymphoproliferative disorder (PTLD), caused in most cases by Epstein-Barr virus infection. These issues of infection and malignancy post-transplant have received a lot of attention as we gave our patients more immunosuppression in the hopes of reducing acute rejection rates. His collaborators are numerous. For the prospective clinical trials, his collaborators include pediatric nephrology colleagues at various centers such as Harvard, Johns Hopkins, University of Pennsylvania, UCSF, University of Washington and UAB. For his epidemiological studies, he collaborates with an adult nephrologist in the Army, with biostatisticians and epidemiologists at Emmes Corporation and with a biostatistician at UNOS.

Michael Eadon, MD, PhD
Associate Professor of Medicine
Indiana University School of Medicine, Indianapolis, Indiana
Michael Eadon, MD, PhD is a translational physician scientist with accomplishments in the realms of pharmacogenomic discovery, construction of a molecular atlas of the kidney in health and disease, and implementation of pharmacogenomics and genomics of chronic kidney disease. He received his MD from Rush University (2006), completed internal medicine residency at Baylor College of Medicine (2009), and completed a combined research fellowship in nephrology, glomerulonephritis and clinical pharmacology at University of Chicago (2013). He joined Indiana University in 2013 and has appointments in nephrology, clinical pharmacology and medical & molecular genetics.
Eadon’s primary research efforts focus on the translation of pharmacogenomics into clinical practice as well as the identification of novel predictors of renal injury from large genomic and transcriptomic datasets. Major focus areas include the evaluation of renal disease expression patterns with spatial transcriptomics and understanding genetic variants that affect this expression (expression quantitative trait loci). He is an active member of NIDDK’s Kidney Precision Medicine Project and the Human BioMolecular Atlas Project, integrating diverse orthogonal datasets to characterize molecular patterns of kidney disease in subjects who have underwent a kidney biopsy.

Joseph P. Gaut, MD, PhD
Ladenson Professor of Pathology, Pathology and Immunology
Division Chief, Anatomic and Molecular Pathology
Washington University in St. Louis School of Medicine
Joseph Gaut, MD, PhD is the Ladenson Professor of Pathology and Immunology, the Division Chief of Anatomic and Molecular Pathology, and the Medical Director of the Barnes-Jewish Hospital Histology Laboratory. Gaut received his MD and PhD degrees from Washington University School of Medicine. He completed an internship in General Surgery at Barnes Hospital/Washington University and a post-doctoral fellowship at Washington University with Jack Ladenson, PhD. He completed his Anatomic Pathology residency at Massachusetts General Hospital, Clinical Pathology residency at Washington University, and fellowships in renal and surgical pathology at Washington University. Gaut’s clinical focus is in the area of renal pathology. His research is focused on improved diagnostic methods for renal disease. He has published extensively in genetic glomerular disease, acute kidney injury (AKI) pathology, and emerging AKI biomarkers. More recently, Gaut has investigated image analysis methods for evaluation of organ quality prior to transplantation. He has collaborated on several consortia projects including the Kidney Precision Medicine Project and the Human Biomolecular Atlas Program.

Gloria Pryhuber
Professor of Pediatrics, Neonatology
University of Rochester Medical Center, Rochester, New York
Gloria Pryhuber is a physician-scientist, currently Professor of Pediatrics (Neonatology) and Environmental Medicine at the University of Rochester Trained at the University for Cincinnati, Cincinnati Children’s Hospital Medical Center Pulmonary Biology Division. She has actively studied pathogenesis of chronic lung disease, especially following premature birth, for more than 25 years. In addition to leadership in clinical-translational studies, she is the PI for Phase I and Phase II of the Lung Development Molecular Atlas Program Human Tissue Core (LungMAP HTC) and now the HuBMAP-Lung Tissue Mapping Center. In partnership with the UNOS national transplant network, using low post-mortem interval, transplant organ recovery protocols, she created and manages the unique BioRepository for Investigation of Neonatal Diseases of the Lung (BRINDL), containing consented, transplant-quality, biospecimens of pediatric research tissues including trachea and lung tissue, peripheral blood mononuclear cells (PBMC), lymph node, spleen and thymus, and has provided over 40 research laboratories with unique opportunities to explore the developing human respiratory tract and immune system in an unusually holistic manner. HTC BRINDL samples have been used and published, by Dr. Pryhuber’s laboratory and by collaborators, for flow and mass cytometry, single cell and nuclear transcriptomics, epigenomics, unbiased mass spectrometry lipid and protein identification, highly multiplexed immunofluorescence and spatial transcriptomics. Most recently, in collaboration with Dr. Jain, she brings this infrastructure and capability to the PCEN pKidBIO Biospecimens Core for biobanking of pediatric human kidney, ureter and bladder, enthusiastic to support an outstanding team of scientists, students and clinical subspecialists in urology and nephrology.

Michael I. Rauchman, MDCM
Chromalloy Professor of Medicine, Nephrology
Washington University in St. Louis School of Medicine
Michael Rauchman is a Professor of Medicine, Pediatrics and Developmental Biology at Washington University School of Medicine. He also serves as Section Chief of Nephrology at the St. Louis VA and directs a pediatric-to-adult transition clinic for kidney disease at Washington University. The main goal of his research is to investigate gene regulation during kidney development and disease. His lab applies this knowledge to understand birth defects of the kidney and to develop treatment strategies to promote repair and regeneration of the kidney in disease states. Ongoing studies are focusing on understanding how transcription factors and their associated chromatin remodeling complexes regulate cell fate of nephron progenitor cells during formation of the kidney. To identify novel genetic causes of congenital anomalies of the kidney and urinary tract (CAKUT), his research group and their collaborators are performing whole exome sequencing of parent-offspring trios comprising a child (fetus or newborn) with severe kidney defects identified on antenatal ultrasound. A second area of emphasis is to investigate epigenetic regulatory mechanisms of the kidney in response to acute and chronic disease. These studies are performed in mouse models and human tissues in collaboration with KPMP.
Advisory committee

Nicholas O. Davidson, MD, DSc
Professor of Medicine
Division Chief, Gastroenterology
Washington University in St. Louis School of Medicine

Christina A. Gurnett, MD, PhD
Professor of Neurology
Division Chief, Pediatric and Developmental Neurology
Washington University in St. Louis School of Medicine